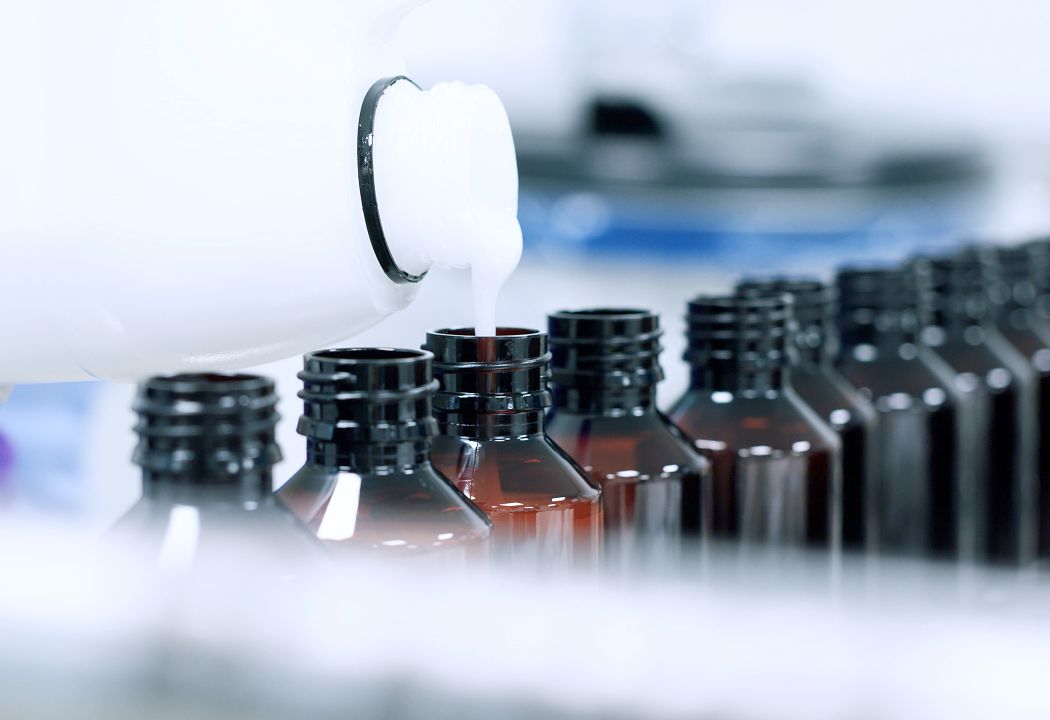
Making Medicine
Solid expertise & flexible solutions
Our expertise spans from pre-clinical development to commercial supply, with a focus on creams, ointments, and nasal sprays.
At Tiofarma, we are committed to delivering comprehensive CDMO services tailored to the unique needs of pharmaceutical companies. From pre-formulation to commercial supply, we provide reliable and flexible solutions to support your product’s journey from concept to market.
Our facilities
Our state-of-the-art manufacturing facility in Oud-Beijerland, the Netherlands, holds both EU-GMP and FDA licenses, ensuring the highest standards of quality and compliance. Complementing this is our modern R&D facility, featuring 400 m² of non-GMP clean rooms for formulation and process development, equipped with laboratory and pilot-scale equipment ranging from a few hundred milliliters to 60 liters. Additionally, we have an in-house analytical laboratory equipped with advanced physicochemical instruments.


Commitment to quality
At Tiofarma, we are committed to maintaining the highest standards of quality and compliance across all our facilities. Our integrated approach ensures that we can provide reliable, flexible, and innovative solutions to meet the unique needs of our clients in the pharmaceutical industry.
Tiofarma in numbers
20
+Chemical engineers, Physicists and Pharmacists
70
+Analyticial chemists and scientists
1994
The start of Tiofarma
50
+Succesful Technical transfers